1. Introduction
In the course of the
SARS-CoV2 pandemic, new regulatory frameworks were put in place that
allowed for the expedited review of data and admission of new vaccines
without safety data [1].
Many of the new vaccines use completely new technologies that have
never been used in humans before. The rationale for this action was that
the pandemic was such a ubiquitous and dangerous threat that it
warrants exceptional measures. In due course, the vaccination campaign
against SARS-CoV2 has started. To date (18 June 2021), roughly 304.5
million vaccination doses have been administered in the EU (https://qap.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#distribution-tab
(accessed on 18 June 2021)), mostly the vector vaccination product
developed by the Oxford vaccination group and marketed by AstraZeneca,
Vaxzevria [2] (approximately 25% coverage in the EU), the RNA vaccination product of BioNTec marketed by Pfizer, Comirnaty [3,4] (approximately 60%), and the mRNA vaccination product developed by Moderna [5]
(approximately 10%). Others account for only around 5% of all
vaccinations. As these vaccines have never been tested for their safety
in prospective post-marketing surveillance studies, we thought it useful
to determine the effectiveness of the vaccines and to compare them with
the costs in terms of side effects.
2. Methods
We used a large Israeli field study [6]
that involved approximately one million persons and the data reported
therein to calculate the number needed to vaccinate (NNTV) to prevent
one case of SARS-CoV2 infection and to prevent one death caused by
COVID-19. In addition, we used the most prominent trial data from
regulatory phase 3 trials to assess the NNTV [4,5,7].
The NNTV is the reciprocal of the absolute risk difference between risk
in the treated group and in the control group, expressed as decimals.
To give an artificial example: An absolute risk difference between a
risk of 0.8 in the control group and a risk of 0.3 in the treated group
would result in an absolute risk difference of 0.5; thus, the number
needed to treat or the NNTV would be 1/0.5 = 2. This is the clinical
effectiveness of the vaccine.
We checked the Adverse Drug Reaction (ADR) database of the European Medicine Agency (EMA: http://www.adrreports.eu/en/search_subst.html#,
accessed on 28 May 2021; the COVID-19 vaccines are accessible under “C”
in the index). Looking up the number of single cases with side effects
reported for the three most widely used vaccines (Comirnaty by
BioNTech/Pfizer, the vector vaccination product Vaxzevria marketed by
AstraZeneca, and the mRNA vaccine by Moderna) by country, we discovered
that the reporting of side effects varies by a factor of 47 (Figure 1).
While the European average is 127 individual case safety reports
(ICSRs), i.e., cases with side effect reports, per 100,000 vaccinations,
the Dutch authorities have registered 701 reports per 100,000
vaccinations, while Poland has registered only 15 ISCRs per 100,000
vaccinations. Assuming that this difference is not due to differential
national susceptibility to vaccination side effects, but due to
different national reporting standards, we decided to use the data of
the Dutch national register (https://www.lareb.nl/coronameldingen;
accessed on 29 May 2021) to gauge the number of severe and fatal side
effects per 100,000 vaccinations. We compare these quantities to the
NNTV to prevent one clinical case of and one fatality by COVID-19.
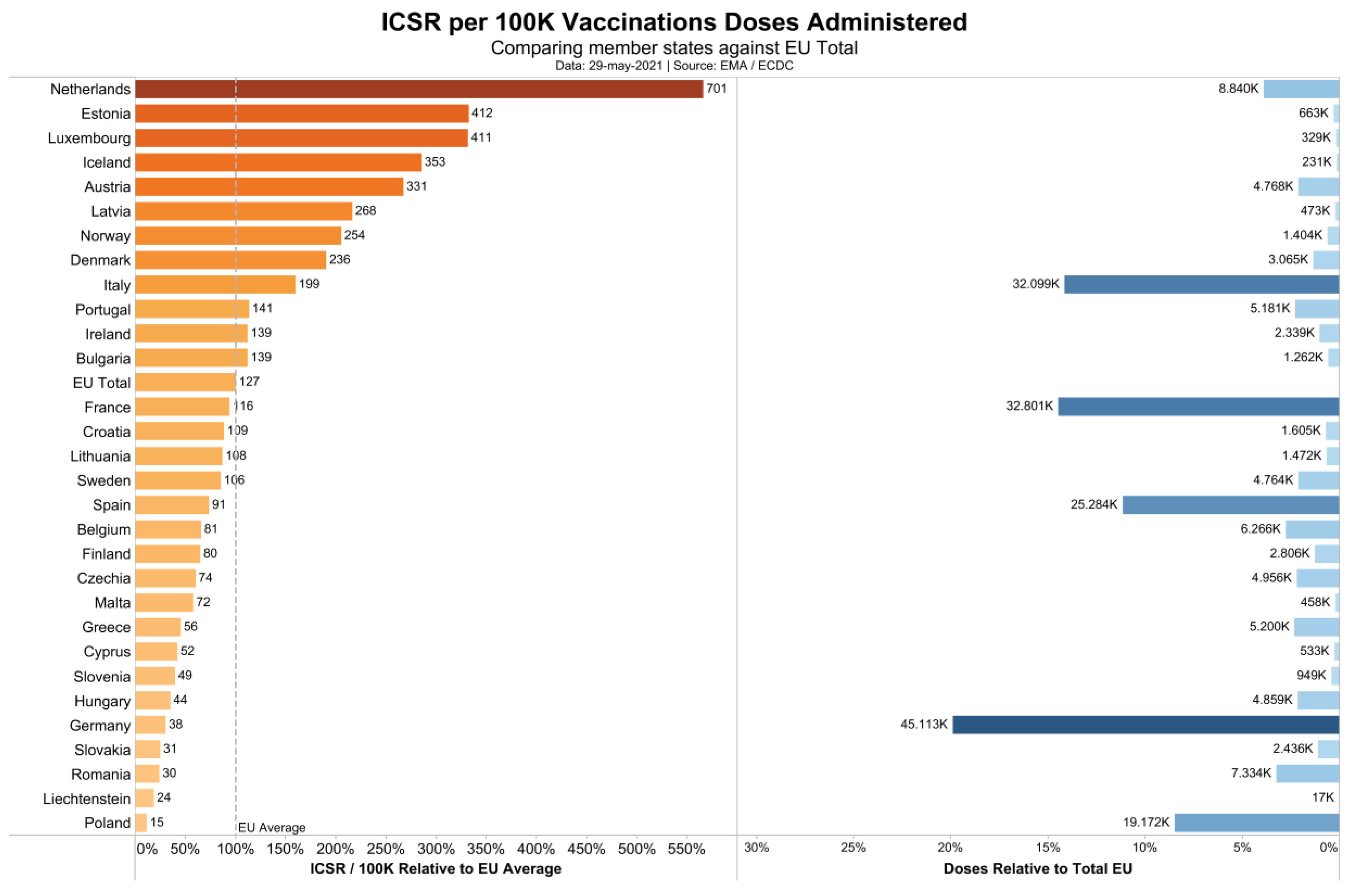
Figure 1.
Individual safety case reports in association with COVID 19 vaccines in Europe.
3. Results
Cunningham was the first
to point out the high NNTV in a non-peer-reviewed comment: Around 256
persons needed to vaccinate with the Pfizer vaccine to prevent one case [8]. A recent large field study in Israel with more than a million participants [6],
where Comirnaty, the mRNA vaccination product marketed by Pfizer, was
applied allowed us to calculate the figure more precisely. Table 1
presents the data of this study based on matched pairs, using
propensity score matching with a large number of baseline variables, in
which both the vaccinated and unvaccinated persons were still at risk at
the beginning of a specified period [6]. We mainly used the estimates from Table 1,
because they are likely closer to real life and derived from the
largest field study to date. However, we also report the data from the
phase 3 trials conducted for obtaining regulatory approval in Table 2 and used them for a sensitivity analysis.
Table 1.
Risk differences and number needed to vaccinate (NNTV) to prevent one
infection, one case of symptomatic illness, and one death from COVID-19.
Data from Dagan et al. [6], N = 596,618 in each group.
| | Documented Infection | Symptomatic Illness | Death from COVID-19 | |||
|---|---|---|---|---|---|---|
| Period | Risk Difference (No./1000 Persons) (95% CI) | NNTV (95% CI) | Risk Difference (No./1000 Persons) (95% CI) | NNTV (95% CI) | Risk Difference (No./1000 Persons) (95% CI) | NNTV (95% CI) |
| 14–20 days after first dose | 2.06 (1.70–2.40) | 486 (417–589) | 1.54 (1.28–1.80) | 650 (556–782) | 0.03 (0.01–0.07) | 33,334 (14,286–100,000) |
| 21–27 days after first dose | 2.31 (1.96–2.69) | 433 (372–511) | 1.34 (1.09–1.62) | 747 (618–918) | 0.06 (0.02–0.11) | 16,667 (9091–50,000) |
| 7 days after second dose to end of follow-up | 8.58 (6.22–11.18) | 117 (90–161) | 4.61 (3.29–6.53) | 217 (154–304) | NA | NA |
Data taken from Table 2 in Dagan et al.’s work. NNTV = 1/risk difference.
Table 2.
Number needed to vaccinate (NNTV) calculated from pivotal phase 3
regulatory trials of the SARS-CoV2 mRNA vaccines of Moderna,
BioNTech/Pfizer, and Sputnik (the vector vaccine of Astra-Zeneca is not
contained here, as the study [9] was active-controlled and not placebo-controlled).
| Vaccine | N Participants Vaccine Group | N Participants Placebo Group | CoV2 Positive End of Trial Vaccine Group | CoV2 Positive End of Trial Placebo Group | Absolute Risk Difference (ARD) | Number Needed to Vaccinate 1/ARR |
|---|---|---|---|---|---|---|
| Moderna [5] $ | 15,181(14,550 *) | 15,170 (14,598 *) | 19 (0.13%) 1 | 269 (1.77%) 1 | 0.0165 | 61 |
| Comirnaty (BioNTech/Pfizer) [4] $ | 18,860 | 18,846 | 8 (0.042%) 2 | 162 (0.86%) 2 | 0.00817 | 123 |
| Sputnik V [7] § | 14,964 | 4902 | 13 (0.087%) **,3 | 47 (1%) **,3 | 0.0091 | 110 |
*
Modified intention to treat-population—basis for calculation; ** taken
from the publication because of slightly different case numbers; $ outcome was a symptomatic COVID-19 case; § outcome was a confirmed infection by PCR-test; 1 after 6 weeks; 2 after 4 weeks; 3 after 3 weeks.
It should be noted that in the Israeli field study, the
cumulative incidence of the infection, visible in the control group
after seven days, was low (Kaplan–Meier estimate <0.5%; Figure 2 in
Dagan et al.’s work [6])
and remained below 3% after six weeks. In the other studies, the
incidence figures after three to six weeks in the placebo groups were
similarly low, between 0.85% and 1.8%. The absolute infection risk
reductions given by Dagan et al. [6]
translated into an NNTV of 486 (95% CI, 417–589) two to three weeks
after the first dose, or 117 (90–161) after the second dose until the
end of follow-up to prevent one documented case (Table 1). Estimates of NNTV to prevent CoV2 infection from the phase 3 trials of the most widely used vaccination products [3,4,5] were between 61 (Moderna) and 123 (Table 2) and were estimated to be 256 by Cunningham [8]. However, it should also be noted that the outcome “Documented infection” in Table 1 refers to CoV2 infection as defined by a positive PCR test, i.e., without considering false-positive results [10],
so that the outcome “symptomatic illness” may better reflect vaccine
effectiveness. If clinically symptomatic COVID-19 until the end of
follow-up was used as an outcome, the NNTV was estimated as 217 (95% CI,
154–304).
In the Israeli field study, 4460 persons in the
vaccination group became infected during the study period and nine
persons died, translating into an infection fatality rate (IFR) of 0.2%
in the vaccination group. In the control group, 6100 became infected and
32 died, resulting in an IFR of 0.5%, which is within the range found
by a review [11].
Using the data from Table 1,
we calculated the absolute risk difference to be 0.00006 (ARD for
preventing one death after three to four weeks), which translates into
an NNTV of 16,667. The 95% confidence interval spanned the range from
9000 to 50,000. Thus, between 9000 and 50,000 people need to be
vaccinated, with a point-estimate of roughly 16,000, to prevent one
COVID-19-related death.
For the other studies listed in Table 2, in the case that positive infection was the outcome [7],
we calculated the NNTV to prevent one death using the IFR estimate of
0.5%; in the case that clinically positive COVID-19 was the outcome [4,5],
we used the case fatality rate estimated as the number of worldwide
COVID-19 cases divided by COVID-19 related deaths, which was 2% (https://www.worldometers.info/coronavirus/
(accessed on 29 May 2021)). In the case of the Sputnik vaccine, one
would thus have to vaccinate 22,000 people to prevent one death. In the
case of the Moderna vaccine, one would have to vaccinate 3050 people to
prevent one death. In the case of Comirnaty, the Pfizer vaccine, 6150
vaccinated people would prevent one death, although using the figure by
Cunningham [8], it would be 12,300 vaccinations to prevent one death.
The side effects data reported in the Dutch register (www.lareb.nl/coronameldingen (accessed on 27 May 2021)) are given in Table 3.
Table 3.
Individual case safety reports for the most widely distributed COVID-19 vaccines according to the Dutch side effects register (www.lareb.nl/coronameldingen (accessed on 29 May 2021)), the absolute numbers per vaccine, and standardization per 100,000 vaccinations.
| General Number of Reports (1) | Serious Side Effects (1) | Deaths (2) | Number of Vaccinations According to (3) | Number of Vaccinations According to ECDC (4) | |
|---|---|---|---|---|---|
| Comirnaty (Pfizer) | 21,321 | 864 | 280 | 5,946,031 | 6,004,808 |
| Moderna | 6390 | 114 | 35 | 531,449 | 540,862 |
| Vaxzevria (AstraZeneca) | 29,865 | 411 | 31 | 1,837,407 | 1,852,996 |
| Janssen | 2596 | 7 | - | 142,069 | 143,525 |
| Unknown | 129 | 15 | 5 | - | 540 |
| Total | 60,301 | 1.411 | 351 | 8,456,956 | 8,542,731 |
| Per 100,000 vaccinations according to Dutch data | 713.03 | 16.68 | 4.15 | | |
| Per 100,000 vaccinations according to ECDC | 705.87 | 16.52 | 4.11 | | |
(1) https://www.lareb.nl/coronameldingen. (2) https://www.lareb.nl/pages/update-van-bijwerkingen. (3) https://coronadashboard.rijksoverheid.nl/landelijk/vaccinaties. (4) https://www.ecdc.europa.eu/en/publications-data/data-covid-19-vaccination-eu-eea. All sites accessed on 27 May 2021. The Dutch government reported two numbers; we took the calculated amounts.
Thus, we need to accept that around 16 cases will develop
severe adverse reactions from COVID-19 vaccines per 100,000 vaccinations
delivered, and approximately four people will die from the consequences
of being vaccinated per 100,000 vaccinations delivered. Adopting the
point estimate of NNTV = 16,000 (95% CI, 9000–50,000) to prevent one
COVID-19-related death, for every six (95% CI, 2–11) deaths prevented by
vaccination, we may incur four deaths as a consequence of or associated
with the vaccination. Simply put: As we prevent three deaths by
vaccinating, we incur two deaths.
The risk–benefit ratio looks
better if we accept the stronger effect sizes from the phase 3 trials.
Using Cunningham’s estimate of NNTV = 12,300, which stems from a
non-peer reviewed comment, we arrived at eight deaths prevented per
100,000 vaccinations and, in the best case, 33 deaths prevented by
100,000 vaccinations. Thus, in the optimum case, we risk four deaths to
prevent 33 deaths, a risk–benefit ratio of 1:8. The risk–benefit ratio
in terms of deaths prevented and deaths incurred thus ranges from 2:3 to
1:8, although real-life data also support ratios as high as 2:1, i.e.,
twice as high a risk of death from the vaccination compared to COVID-19,
within the 95% confidence limit.
4. Discussion
The
COVID-19 vaccines are immunologically effective and can—according to
the publications—prevent infections, morbidity, and mortality associated
with SARS-CoV2; however, they incur costs. Apart from the economic
costs, there are comparatively high rates of side effects and
fatalities. The current figure is around four fatalities per 100,000
vaccinations, as documented by the most thorough European documentation
system, the Dutch side effects register (lareb.nl). This tallies well
with a recently conducted analysis of the U.S. vaccine adverse reactions
reporting system, which found 3.4 fatalities per 100,000 vaccinations,
mostly with the Comirnaty (Pfizer) and Moderna vaccines [12].
Is
this a few or many? This is difficult to say, and the answer is
dependent on one’s view of how severe the pandemic is and whether the
common assumption that there is hardly any innate immunological defense
or cross-reactional immunity is true. Some argue that we can assume
cross-reactivity of antibodies to conventional coronaviruses in 30–50%
of the population [13,14,15,16]. This might explain why children and younger people are rarely afflicted by SARS-CoV2 [17,18,19]. An innate immune reaction is difficult to gauge. Thus, low seroprevalence figures [20,21,22]
may not only reflect a lack of herd immunity, but also a mix of
undetected cross-reactivity of antibodies to other coronaviruses, as
well as clearing of infection by innate immunity.
However, one
should consider the simple legal fact that a death associated with a
vaccination is different in kind and legal status from a death suffered
as a consequence of an incidental infection.
Our data should be viewed in the light of its inherent limitations:
The
study which we used to gauge the NNTV was a single field study, even
though it is the largest to date. The other data stem from regulatory
trials that were not designed to detect maximum effects. The field study
was somewhat specific to the situation in Israel, and studies in other
countries and other populations or other post-marketing surveillance
studies might reveal more beneficial clinical effect sizes when the
prevalence of the infection is higher. This field study also suffered
from some problems, as a lot of cases were censored due to unknown
reasons, presumably due to a loss to follow-up. However, the regulatory
studies compensate for some of the weaknesses, and thereby generate a
somewhat more beneficial risk–benefit ratio.
The ADR database of the EMA collects reports of different kinds, by doctors, patients, and authorities. We observed (Figure 1)
that the reporting standards vary hugely across countries. It might be
necessary for the EMA and for national governments to install better
monitoring procedures in order to generate more reliable data. Some
countries have tight reporting schemes, some report in a rather loose
fashion. As we have to assume that the average number of side effects is
roughly similar across countries, we would expect a similar reporting
quota. However, when inspecting the reports according to countries, we
can see a large variance. Our decision to use the Dutch data as a proxy
for Europe was derived from this discovery. One might want to challenge
this decision, but we did not find any data from other countries being
more valid than those used here. Apart from this, our data tallied well
with the data from the U.S. CDC vaccine adverse reporting system [12], which indirectly validates our decision.
One
might argue that it is always difficult to ascertain causality in such
reports. This is certainly true; however, the Dutch data, especially the
fatal cases, were certified by medical specialists (https://www.lareb.nl/media/eacjg2eq/beleidsplan-2015-2019.pdf (accessed on 29 May 2021)), page 13: “All
reports received are checked for completeness and possible ambiguities.
If necessary, additional information is requested from the reporting
party and/or the treating doctor The report is entered into the database
with all the necessary information. Side effects are coded according to
the applicable (international) standards. Subsequently an individual
assessment of the report is made. The reports are forwarded to the
European database (Eudravigilance) and the database of the WHO
Collaborating Centre for International Drug Monitoring in Uppsala. The
registration holders are informed about the reports concerning their
product.”).
A recent experimental study showed that the SARS-CoV2 spike protein is sufficient to produce endothelial damage [23].
This provides a potential causal rationale for the most serious and
most frequent side effects, namely, vascular problems such as thrombotic
events. The vector-based COVID-19 vaccines can produce soluble spike
proteins, which multiply the potential damage sites [24].
The spike protein also contains domains that may bind to cholinergic
receptors, thereby compromising the cholinergic anti-inflammatory
pathways, enhancing inflammatory processes [25].
A recent review listed several other potential side effects of COVID-19
mRNA vaccines that may also emerge later than in the observation
periods covered here [26].
In
the Israeli field study, the observation period was six weeks, and in
the U.S. regulatory studies between four to six weeks, a period commonly
assumed to be sufficient to see a clinical effect of a vaccine, because
it would also be the time frame within which someone who was infected
initially would fall ill and perhaps die. Had the observation period
been longer, the clinical effect size might have increased, i.e., the
NNTV could have become lower and, consequently, the ratio of benefit to
harm could have increased in favor of the vaccines. However, as noted
above, there is also the possibility of side effects developing with
some delay and influencing the risk–benefit ratio in the opposite
direction [26]. This should be studied more systematically in a long-term observational study.
Another
point to consider is that initially, mainly older persons and those at
risk were entered into the national vaccination programs. It is to be
hoped that the tally of fatalities will become lower as a consequence of
the vaccinations, as the age of those vaccinated decreases.
However,
we do think that, given the data, we should not wait to see whether
more fatalities accrue, but instead use the data available to study who
might be at risk of suffering side effects and pursue a diligent route.
Finally,
we note that from experience with reporting side effects from other
drugs, only a small fraction of side effects is reported to adverse
events databases [27,28]. The median underreporting can be as high as 95% [29].
Given
this fact and the high number of serious side effects already reported,
the current political trend to vaccinate children who are at very low
risk of suffering from COVID-19 in the first place must be reconsidered.
5. Conclusions
The
present assessment raises the question whether it would be necessary to
rethink policies and use COVID-19 vaccines more sparingly and with some
discretion only in those that are willing to accept the risk because
they feel more at risk from the true infection than the mock infection.
Perhaps it might be necessary to dampen the enthusiasm by sober facts?
In our view, the EMA and national authorities should instigate a safety
review into the safety database of COVID-19 vaccines and governments
should carefully consider their policies in light of these data.
Ideally, independent scientists should carry out thorough case reviews
of the very severe cases, so that there can be evidence-based
recommendations on who is likely to benefit from a SARS-CoV2 vaccination
and who is in danger of suffering from side effects. Currently, our
estimates show that we have to accept four fatal and 16 serious side
effects per 100,000 vaccinations in order to save the lives of 2–11
individuals per 100,000 vaccinations, placing risks and benefits on the
same order of magnitude.